Fight Retinal Blindness!
The FRB! Project serves as the foundation of the Save Sight Registries. Designed to collect high quality outcomes data from patients in the clinical setting, it tracks the effectiveness and safety of new and emerging treatments for Age-Related Macular Degeneration, Diabetic Retinopathy and Retinal Vein Occlusion which are among the commonest causes of vision impairment and blindness world-wide.
Retinal Diseases
The Fight Retinal Blindness! (FRB!) project is the flagship project the Save Sight Registries that has spun off all the other Save Sight Registries modules. One of the most advanced ophthalmic web-based registries in the world, it is a unique and sophisticated platform for tracking the long-term effectiveness and safety of treatments for retinal (mostly macular) diseases. It includes three registries of treatment outcomes: Age-Related Macular Degeneration (wet and dry), Diabetic Macular Oedema and Retinal Vein Occlusion
Registry users can participate in national and international audits, receive invitations to contribute data and co-author publications and publish their own data.
All users receive training via online modules or video conference. Support is free and available at the user’s convenience.
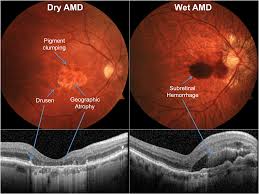
Age-Related Macular Degeneration
The AMD Registry is the first module of the FRB! Project. The registry mainly tracks the outcomes of treatment of eye injections for wet (or neovascular) AMD. A new feature was added in 2023 which allows doctors to track the outcomes of the new treatments for advanced dry, or atrophic” AMD
Established in 2008, the AMD module of the Fight Retinal Blindness Registry has steadily grown, with encouraging clinician uptake across Australia and 20 countries around the world. The Fight Retinal Blindness Registry is now recognized as one of the world’s leading observational databases in macular disease. The dataset allows us to determine the correct timing and choice of treatment for patients with macular disease.
A sophisticated interface allows clinicians to capture the patient’s response on the perceived effect of treatment on their quality of life for all 3 FRB! modules. This is done simply by the patient entering their responses directly into a tablet or any hand-held device. The responses are then automatically and securely transmitted to the database

Diabetic Macular Oedema
The DME Module of the FRB! registry has tracked treatment outcomes for DME, the commonest cause of loss of vision in people with diabetes, since 2009. Like the other FRB! modules, one of the main interests of analyses of these data is whether new treatments perform as well as they are supposed to. For example, we were able to establish that aflibercept did actually have a stronger effect on DME (and RVO) after it had been released for general use in 2013
Retinal Vein Occlusion
This module that was established in 2011 is the only international registry that has been specifically designed to track the outcomes of RVO, which is the second commonest vascular disease of the retina after diabetic macular oedema.
The data collected by all 3 FRB! modules allows the clinicians to compare their own patient outcomes with those of their colleagues for benchmarking. Users also have the option to collect patient-reported outcome measures through targeted questionnaires that are also available in Spanish, German and Chinese
Chief Investigator
Mark Gillies
MB BS, PhD, FRANZCO
Director of Research, Save Sight Institute
Dept. of Ophthalmology & Eye Health, University of Sydney
Chief Investigator and Innovator, Save Sight Registries


